Which of the Following Would Cause Entropy to Decrease
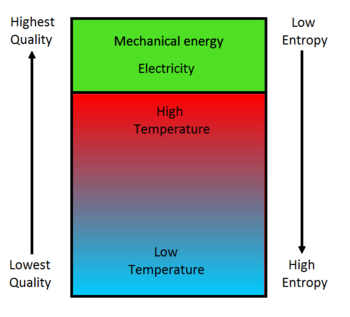
Second Law Of Thermodynamics Energy Education
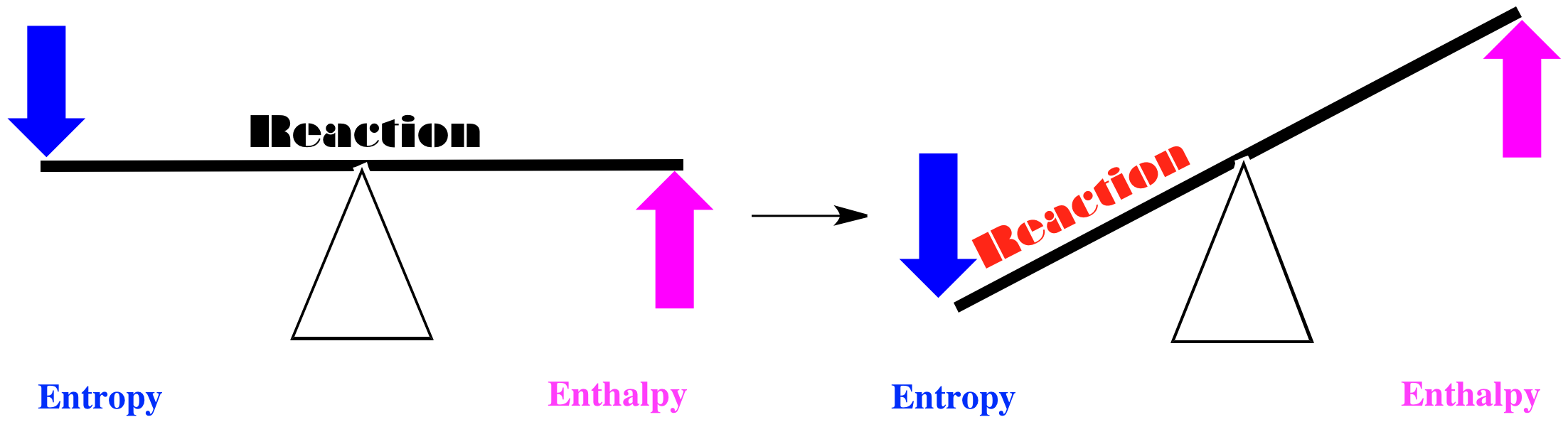

18 3 Entropy And The Second Law Of Thermodynamics Chemistry Libretexts
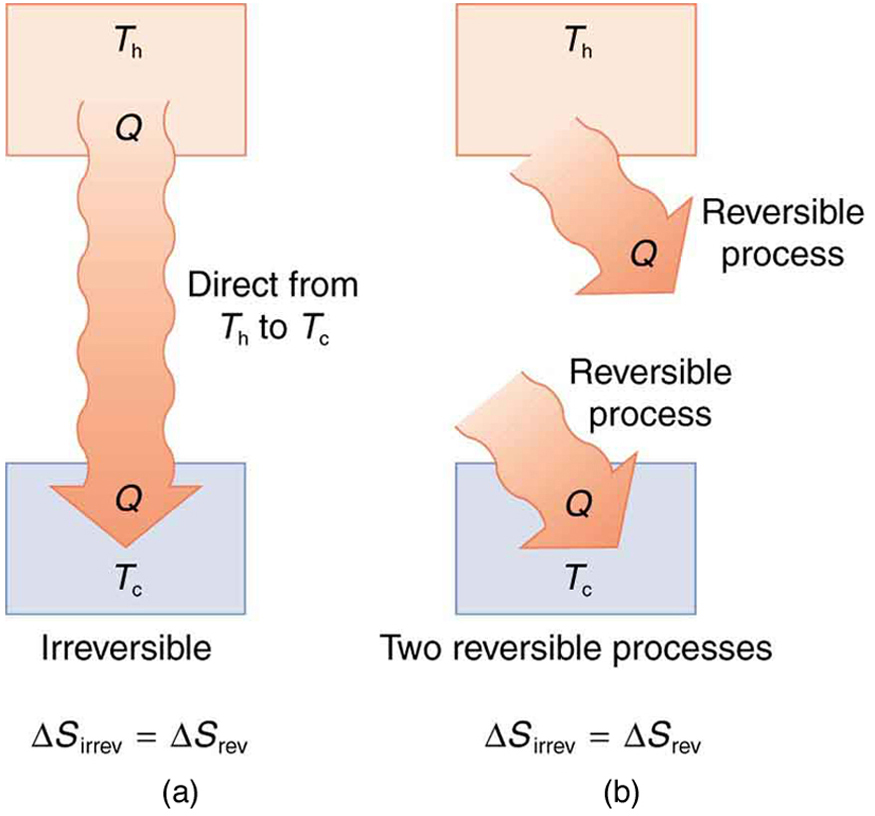
Entropy And The Second Law Of Thermodynamics Disorder And The Unavailability Of Energy College Physics

Entropy And The Second Law Of Thermodynamics Disorder And The Unavailability Of Energy Physics
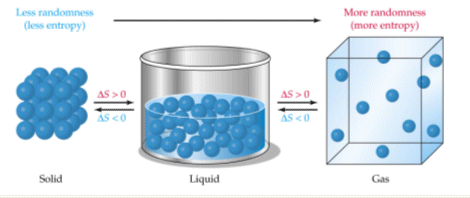
Which Of The Following Processes Shows A Decrease In Entropy Of The System Socratic

Predict In Which Of The Following Entropy Increases Decreases A H 2 G To 2h G B 2nahco 3 To Na 2 Co 3 S Co 2 G H 2 O G C A Liquid Crystallizes Into A Solid D Temperature

Chemical Thermodynamics Ppt Download

Example 17 1 Predicting The Sign Of Entropy Change Ppt Video Online Download

Predict In Which Of The Following Entropy Increases Decreases A A Liquid Crystallizes Into A Solid B Temperature F A Crystalline Solid Is Raised From 0 K To 115 K C 2nahco3 S Nano3

Thermodynamics Does Entropy Increase With A Decrease Or With An Increase In A System S Temperature Physics Stack Exchange
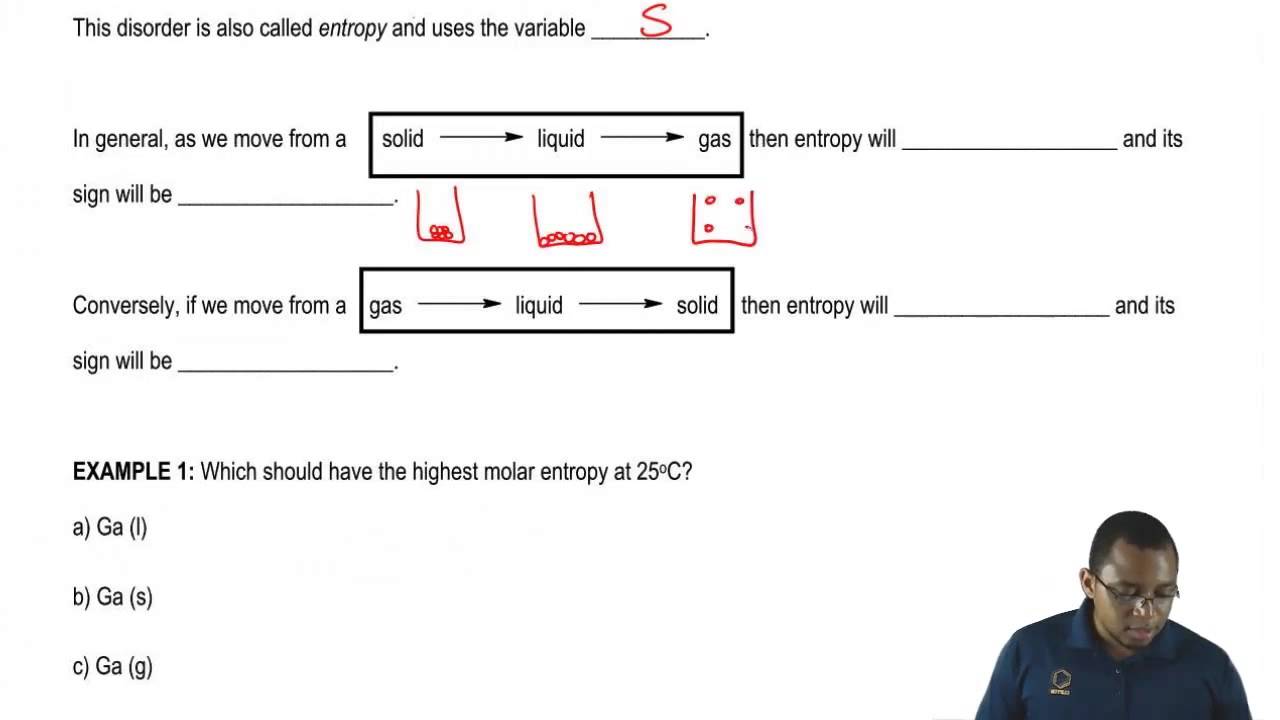
Increasing Or Decreasing Entropy Youtube

Entropy And The Second Law Of Thermodynamics Disorder And The Unavailability Of Energy Physics

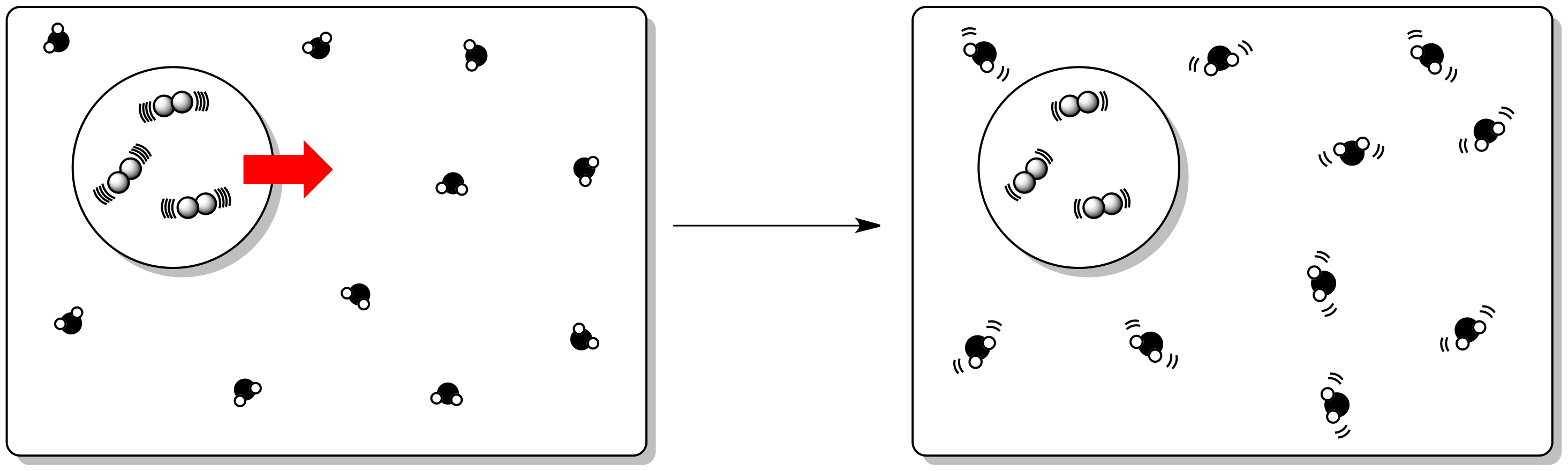
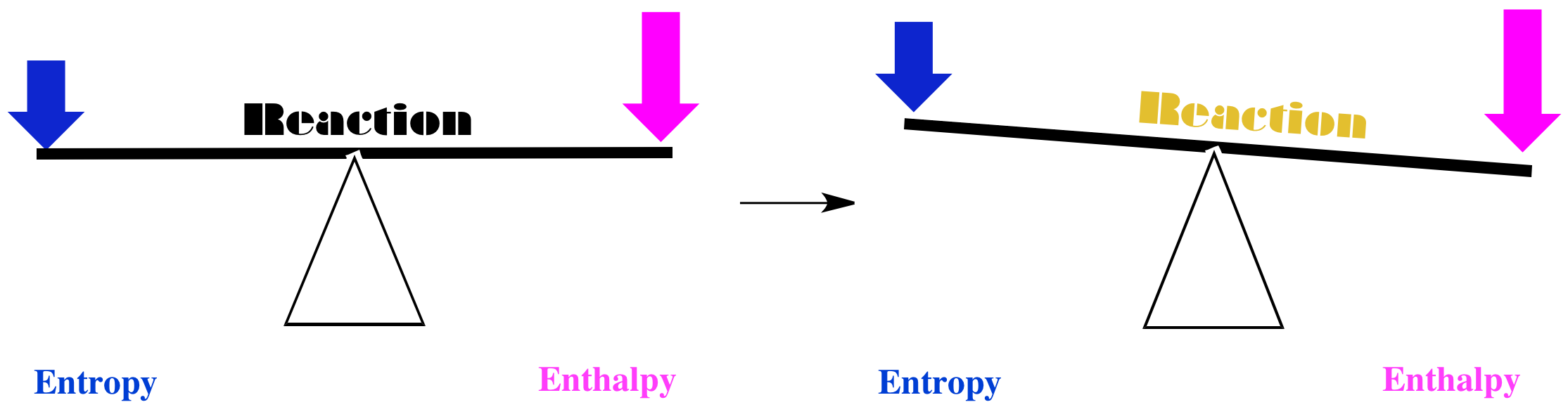


Comments
Post a Comment